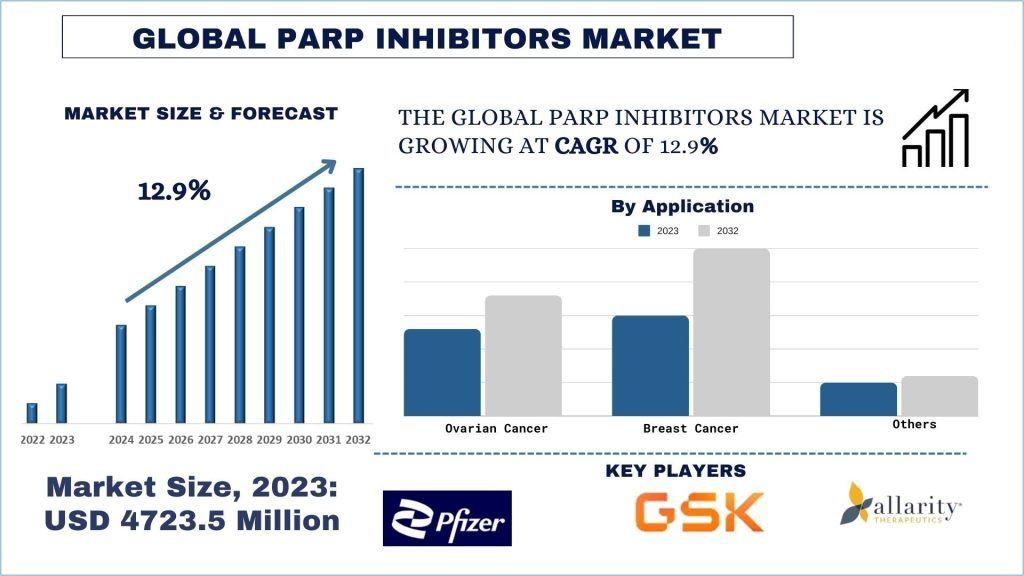
According to UnivDatos, the PARP inhibitors market was valued at USD 4723.5 Million and is expected to grow at a strong CAGR of around 12.9% during the forecast period (2024-2032) owing to investment in PARP inhibitors. The introduction of poly (ADP-ribose) polymerase (PARP) inhibitors has significantly reshaped the cancer treatment landscape in the United States. These targeted therapies have emerged as a cornerstone in the management of multiple cancer types, including ovarian, breast, prostate, and pancreatic cancers—particularly among patients with specific genetic mutations such as BRCA1 and BRCA2.
The rising burden of cancer across the U.S. is a major factor driving the increasing demand for PARP inhibitors and is expected to continue fueling market growth in the coming years. According to the Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI), the U.S. Cancer Statistics (USCS) report for 2020 highlights the scale of this challenge.
Cancer Incidence and Mortality in the United States
- Incidence: In 2020, approximately 1.8 million new cancer cases were diagnosed nationwide, corresponding to an incidence rate of 442 cases per 100,000 people.
- Mortality: During the same year, cancer accounted for nearly 602,350 deaths, translating to a mortality rate of 152 deaths per 100,000 people.
These figures underscore the urgent need for innovative and more effective treatment options, reinforcing the importance of PARP inhibitors in modern oncology.
The Science Behind PARP Inhibitors
PARP inhibitors function by targeting the PARP enzyme, which plays a crucial role in repairing single-strand DNA breaks. When PARP activity is blocked, DNA damage accumulates within cancer cells, ultimately leading to cell death. This effect is particularly pronounced in tumor cells that already have compromised DNA repair mechanisms, such as those harboring BRCA mutations.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/parp-inhibitors-market?popup=report-enquiry
This targeted mechanism of action enables PARP inhibitors to selectively eliminate cancer cells while minimizing damage to healthy tissue, making them a powerful and precision-driven therapeutic option.
Key PARP Inhibitors Approved in the U.S.
Several PARP inhibitors have received regulatory approval in the United States and are actively shaping cancer treatment protocols:
- Olaparib (Lynparza) – AstraZeneca and Merck
- Niraparib (Zejula) – GlaxoSmithKline
- Rucaparib (Rubraca) – Clovis Oncology
- Talazoparib (Talzenna) – Pfizer
Recent Company News and Market Developments
The U.S. PARP inhibitor market has experienced strong momentum in recent months, driven by clinical advancements, regulatory milestones, and strategic collaborations.
AstraZeneca and Merck
- January 2024: The FDA approved Lynparza in combination with bevacizumab for first-line maintenance treatment of advanced ovarian cancer in patients with homologous recombination deficiency (HRD), based on favorable results from the PAOLA-1 trial.
- February 2024: The companies announced an expanded collaboration to explore Lynparza combinations with immunotherapies and targeted agents across prostate and pancreatic cancers.
GlaxoSmithKline
- March 2024: The FDA granted accelerated approval to Zejula in combination with pembrolizumab for platinum-resistant ovarian cancer, supported by Phase II MOONSTONE trial data.
- April 2024: GSK committed additional investments toward next-generation PARP inhibitors and novel combination strategies to address resistance challenges.
Clovis Oncology
- April 2024: The FDA approved Rubraca for metastatic castration-resistant prostate cancer (mCRPC) patients with BRCA mutations, following positive TRITON2 trial outcomes.
- May 2024: The company secured USD 200 million in funding to accelerate PARP inhibitor combination trials and expand its oncology pipeline.
Pfizer
- June 2024: Pfizer initiated a Phase III clinical trial evaluating Talzenna in combination with enzalutamide for mCRPC treatment.
- July 2024: Talzenna received FDA breakthrough therapy designation for use alongside a novel immunotherapy in advanced pancreatic cancer, based on encouraging early-stage trial results.
Impact of PARP Inhibitors on Cancer Treatment
The growing adoption of PARP inhibitors has brought meaningful improvements to cancer care in the U.S., particularly through their role as targeted therapies.
Click here to view the Report Description & TOC: https://univdatos.com/reports/parp-inhibitors-market
Targeted Precision Therapy
PARP inhibitors are designed to exploit specific genetic vulnerabilities in cancer cells, allowing for more precise treatment with reduced toxicity. Compared to traditional chemotherapy, these agents offer improved tolerability and enhanced therapeutic outcomes, representing a major advancement in personalized oncology care.
Conclusion
The expansion of PARP inhibitors in the U.S. oncology market marks a pivotal shift toward more targeted and personalized cancer treatment. Strong clinical trial outcomes, continued regulatory approvals, and rising investments are positioning these therapies as essential components of future cancer care. As ongoing research broadens their clinical applications and addresses resistance mechanisms, PARP inhibitors are expected to deliver improved survival outcomes and renewed hope for cancer patients nationwide.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/